16 exclusive varieties are bright: Jichuan, Yangtze River and Jimin Credible …
▍ Source/intranet ▍ Author/Bai Yu
Up nearly 30%! Beijing stands out from the rest, selling cardiovascular and cerebrovascular diseases, tumors and respiratory systems.
In recent years, Chinese medicine industry policies have been introduced frequently, and it has been more favorable since this year. In February, the General Office of the State Council issued the Notice on Several Policies and Measures for Accelerating the Development of Traditional Chinese Medicine. In June, National Medical Products Administration launched ten key research projects, including the research on the effectiveness and safety evaluation of traditional Chinese medicine and the whole process quality control. Real-world data support the research on evaluation methods of traditional Chinese medicine, rare disease treatment drugs, innovation and clinical urgently needed medical devices; National Health Commission and other three departments issued the Opinions on Further Strengthening the Work of Traditional Chinese Medicine in General Hospitals and Promoting the Coordinated Development of Chinese and Western Medicine. On July 7th, official website City, state administration of traditional chinese medicine issued the notice of "Implementation Plan of Traditional Chinese Medicine Cultural Communication Action (2021-2025)", which proposed four key tasks, such as deepening the essence of traditional Chinese medicine culture, so as to further integrate traditional Chinese medicine into public life. According to industry insiders, the Chinese medicine industry continues to usher in favorable policies. While optimizing the industry structure, accelerating inheritance and innovation is conducive to going abroad and further promoting the development of the Chinese medicine industry.
Quarterly sales of Chinese patent medicines in key cities from 2020 to 2021 Q1
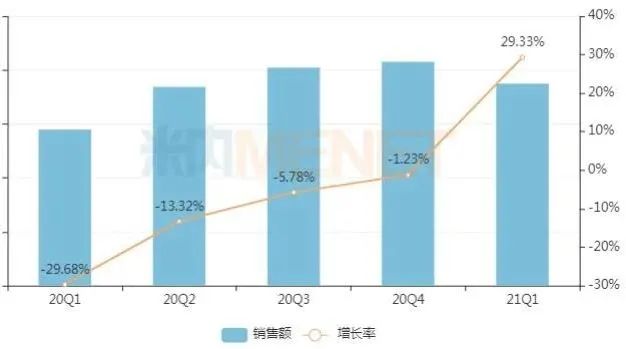
Source: terminal competition pattern of Chinese patent medicines in public hospitals in key cities
According to the data of Minenet, in 2020, the sales of proprietary Chinese medicines in public hospitals in key cities will be nearly 30 billion yuan, down more than 10% year-on-year. Judging from the quarterly sales trend, the market gradually picked up after experiencing the low tide in the first quarter of last year, and rose by 29.33% in the first quarter of this year.
Urban Pattern of Chinese Patent Medicines in Public Hospitals of Key Cities in 2021Q1
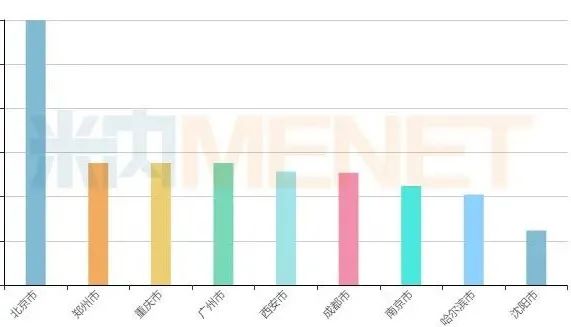
Source: terminal competition pattern of Chinese patent medicines in public hospitals in key cities
Judging from the urban pattern, Beijing stands out, with sales of nearly 1.8 billion yuan; The sales of Zhengzhou, Chongqing and Guangzhou all exceeded 800 million yuan. From the perspective of TOP20 manufacturers, Zhejiang Kanglaite Pharmaceutical, Yangzijiang Pharmaceutical Group, Shanghai Green Valley Pharmaceutical, Hangzhou Zhongmei Huadong Pharmaceutical and Jichuan Pharmaceutical Group are in the top five; The sales growth rate of Lunan Houpu Pharmaceutical, Tibet Qizheng Tibetan Medicine and Jiangsu Kangyuan Pharmaceutical all exceeded 50%.
From the perspective of large-scale pattern, drugs for cardiovascular and cerebrovascular diseases, drugs for tumor diseases and drugs for respiratory diseases are the hottest, accounting for more than 55% of the total market share; Drugs for musculoskeletal diseases, drugs for pediatrics, drugs for invigorating qi and enriching blood, etc. increased by more than 50%.
The Yangtze River and Jichuan … 16 exclusive products "preempted" TOP20, and 4 products rose by over 30%.
TOP10, a Chinese patent medicine product in public hospitals of key cities in 2021Q1
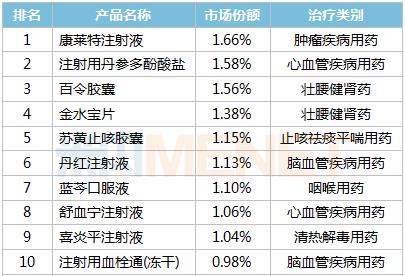
Source: terminal competition pattern of Chinese patent medicines in public hospitals in key cities
Among TOP20 products, except Shuxuening injection, cinobufotalin capsule, Xingnaojing injection and Tongguanteng injection, 16 products such as Kanglaite injection, Danshen Polyphenolate for injection, Jinshuibao tablet, Suhuang Zhike capsule and Pudilan Xiaoyan oral liquid are exclusive products, involving Zhejiang Kanglaite Pharmaceutical, Shanghai Lvgu Pharmaceutical, Jiangxi Jimin Kexin Pharmaceutical, Yangzijiang Pharmaceutical Group Beijing Haiyan Pharmaceutical, Jichuan Pharmaceutical Group and other manufacturers. In terms of dosage forms, injections account for 60%. From the perspective of treatment categories, cancer diseases are the most commonly used drugs, with 5 drugs; Followed by drugs for cerebrovascular diseases, there are 4; There are three drugs for cardiovascular diseases and three drugs for strengthening waist and strengthening kidney respectively.
From the perspective of sales growth, more than 80% of TOP20 products have positive growth, among which Tongguanteng injection has the fastest growth rate, and cinobufotalin capsules, Xueshuantong for injection (freeze-dried) and Jinshuibao tablets all exceed 30%. It is worth mentioning that only three of the TOP20 products in 2020 are positive growth.
Quarterly sales of Jinshuibao tablets in public hospitals in key cities from 2020 to 2021 Q1 (unit: 10,000 yuan)
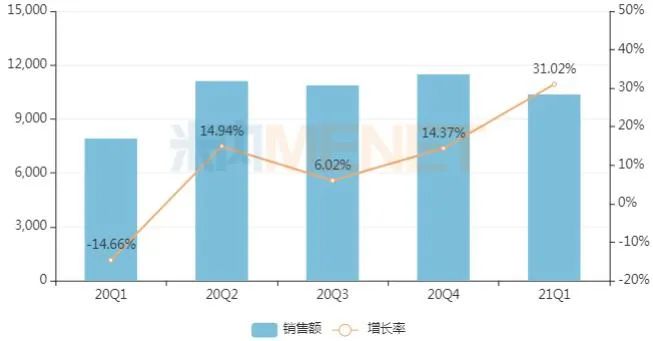
Source: terminal competition pattern of Chinese patent medicines in public hospitals in key cities
Jinshuibao tablets are the exclusive products of Jiangxi Jimin Kexin Pharmaceutical. According to the data of Minenet, the sales of Jinshuibao tablets in public hospitals in key cities have increased rapidly in recent years, from less than 50 million yuan in 2017 to more than 400 million yuan in 2020. The sales in the first quarter of this year have exceeded 100 million yuan, a year-on-year increase of 31.02%.
Quarterly sales of Tongguanteng injection (Xiaoaiping injection) in public hospitals in key cities from 2020 to 2021 Q1 (unit: 10,000 yuan)
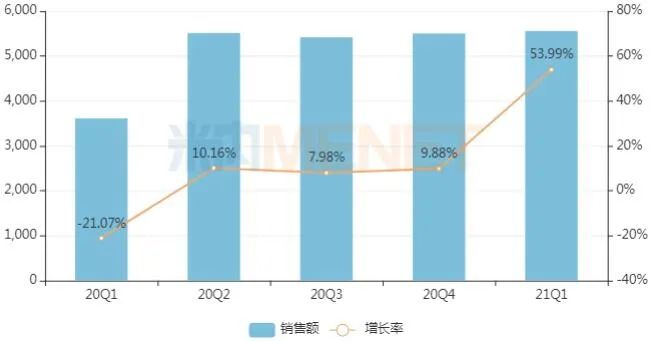
Source: terminal competition pattern of Chinese patent medicines in public hospitals in key cities
Tongguanteng injection (Xiaoaiping injection) is used for the treatment of esophageal cancer, gastric cancer, liver cancer and other tumors, and is combined with radiotherapy and chemotherapy as adjuvant treatment. According to the data in the Minenet, in 2020, the sales of Tongguanteng injection (Xiaoaiping injection) in public hospitals in key cities exceeded 200 million yuan, up 2.25% year-on-year; The first quarter of this year increased by more than 50% year-on-year. At present, the only manufacturers of this product are Nanjing Shenghe Pharmaceutical and Tonghua Jinma Pharmaceutical Group, among which Nanjing Shenghe Pharmaceutical has the largest market share.
The policy is good! Renfu, Kangyuan, Yiling … Class 1 new drugs launched an impact.
Since 2020, a total of six Chinese patent medicines have been approved for listing. Among them, Lianhua Qingke Tablet is a 6.1-class new Chinese medicine developed by Yiling Pharmaceutical under the guidance of collateral disease theory of traditional Chinese medicine for treating acute tracheobronchitis. Jingu Zhitong Gel is a 6.1-class new Chinese medicine developed on the basis of the clinical experience of Professor Sun Shuchun, an orthopedic expert at China Academy of Traditional Chinese Medicine. Kangyuan Pharmaceutical has independent and complete intellectual property rights of this new medicine. The total alkaloid tablets of Ramulus Mori from Beijing Wuhe Boao Pharmaceutical Co., Ltd. are used to treat type 2. In the first half of this year, Qingfei Detoxification Granule of Institute of Clinical Basic Medicine of Chinese Academy of Traditional Chinese Medicine, Huashi Detoxification Granule of Guangdong Yifang Pharmaceutical and Xuanfei Detoxification Granule of Shandong Buchang Pharmaceutical were approved for listing.
Application for listing of Class I new Chinese patent medicines since 2020
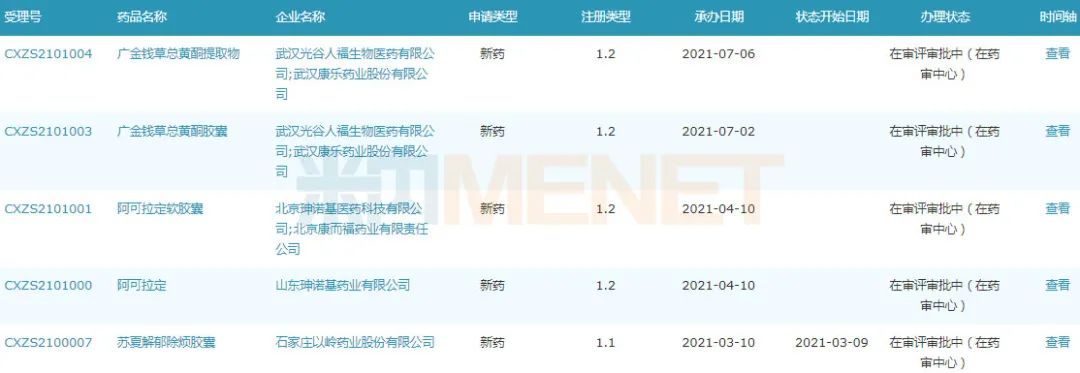
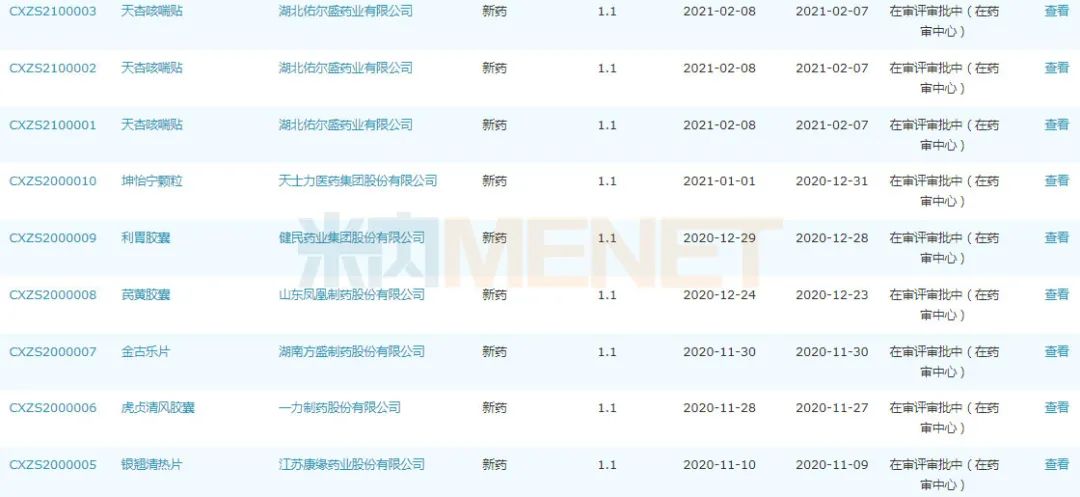
Source: Minenet MED2.0 China Drug Evaluation Database.
In addition, since 2020, a total of 13 Chinese patent medicines have been submitted for listing, among which 10 are Class I new drugs, including Yinqiao Qingre Tablet of Jiangsu Kangyuan Pharmaceutical, Jingule Tablet of Hunan Fangsheng Pharmaceutical, Kunyining Granule of Tasly, Su Xia Jieyu Bufan Capsule of Yiling Pharmaceutical, etc. A few days ago, the total flavonoids capsule of Desmodium styracifolium was submitted to the market as a new class 1.2 Chinese patent medicine and was undertaken by CDE. At present, it is under review and approval, and it is expected to become the first new class 1 Chinese patent medicine in Renfu in recent ten years.
